Abstract
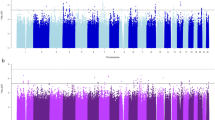
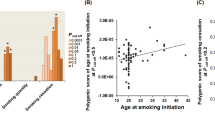
Cigarette smoking is a risk factor for a wide range of human diseases1. To investigate the genetic components associated with smoking behaviours in the Japanese population, we conducted a genome-wide association study of four smoking-related traits using up to 165,436 individuals. In total, we identified seven new loci, including three loci associated with the number of cigarettes per day (EPHX2–CLU, RET and CUX2–ALDH2), three loci associated with smoking initiation (DLC1, CXCL12–TMEM72-AS1 and GALR1–SALL3) and LINC01793–MIR4432HG, associated with the age of smoking initiation. Of these, three loci (LINC01793–MIR4432HG, CXCL12–TMEM72-AS1 and GALR1–SALL3) were found by conducting an additional sex-stratified genome-wide association study. This additional analysis showed heterogeneity of effects between sexes. The cross-sex linkage disequilibrium score regression2,3 analysis also indicated that the genetic component of smoking initiation was significantly different between the sexes. Cross-trait linkage disequilibrium score regression analysis and trait-relevant tissue analysis showed that the number of cigarettes per day has a specific genetic background distinct from those of the other three smoking behaviours. We also report 11 diseases that share genetic basis with smoking behaviours. Although the current study should be carefully considered owing to the lack of replication samples, our findings characterized the genetic architecture of smoking behaviours. Further studies in East Asian populations are warranted to confirm our findings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
GWAS summary statistics of the smoking behaviours are publicly available at our website (JENGER; http://jenger.riken.jp/en/) and the National Bioscience Database Center (NBDC) Human Database (research ID: hum0014) as open data without any access restrictions. The accession numbers for each phenotype in the NBDC Human Database are as follows: age of smoking initiation: hum0014.v14.asi.v1; CPD: hum0014.v14.cpd.v1; smoking initiation: hum0014.v14.ens.v1; smoking cessation: hum0014.v14.fcs.v1. GWAS genotype data from the participants were deposited at the NBDC Human Database (research ID: hum0014).
References
US Department of Health and Human Services. The Health Consequences of Smoking — 50 Years of Progress: A Report of the Surgeon General (CDC, 2014).
Bulik-Sullivan, B. K. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Finucane, H. K. et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat. Genet. 47, 1228–1235 (2015).
Global Health Estimates 2015: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015 (WHO, 2016).
WHO Report on the Global Tobacco Epidemic, 2017: Monitoring Tobacco Use and Prevention Policies (WHO, 2017).
Summary Results of the National Health and Nutrition Survey Japan (National Institute of Health and Nutrition, 2016).
Jamal, A. et al. Current cigarette smoking among adults — United States, 2005–2015. MMWR Morb. Mortal. Wkly Rep. 65, 1205–1211 (2016).
Boardman, J. D., Blalock, C. L. & Pampel, F. C. Trends in the genetic influences on smoking. J. Health Soc. Behav. 51, 108–123 (2010).
Yang, J. & Li, M. D. Converging findings from linkage and association analyses on susceptibility genes for smoking and other addictions. Mol. Psychiatry 21, 992–1008 (2016).
The Tobacco and Genetics Consortium Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat. Genet. 42, 441–447 (2010).
Berrettini, W. et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol. Psychiatry 13, 368–373 (2008).
Kumasaka, N. et al. Haplotypes with copy number and single nucleotide polymorphisms in CYP2A6 locus are associated with smoking quantity in a Japanese population. PLoS One 7, e44507 (2012).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Zheng, J. et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 33, 272–279 (2017).
Thorgeirsson, T. E. et al. Sequence variants at CHRNB3–CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 42, 448–453 (2010).
Liu, J. Z. et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat. Genet. 42, 436–440 (2010).
Nagai, A. et al. Overview of the BioBank Japan Project: study design and profile. J. Epidemiol. 27, S2–S8 (2017).
Hirata, M. et al. Cross-sectional analysis of BioBank Japan clinical data: a large cohort of 200,000 patients with 47 common diseases. J. Epidemiol. 27, S9–S21 (2017).
Kanai, M., Tanaka, T. & Okada, Y. Empirical estimation of genome-wide significance thresholds based on the 1000 Genomes Project data set. J. Hum. Genet. 61, 861–866 (2016).
Masaoka, H. et al. Aldehyde dehydrogenase 2 polymorphism is a predictor of smoking cessation. Nicotine Tob. Res. 19, 1087–1094 (2017).
Masaoka, H. et al. Combination of ALDH2 and ADH1B polymorphisms is associated with smoking initiation: a large-scale cross-sectional study in a Japanese population. Drug Alcohol Depend. 173, 85–91 (2017).
McKay, J. D. et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat. Genet. 49, 1126–1132 (2017).
McVean, G. Linkage disequilibrium. in Handbook of Statistical Genetics. 3rd ed. (eds. Balding, D.J., Bishop, M., & Cannings, C.) 914–918 (Wiley, 2007).
Patel, Y. M. et al. Novel association of genetic markers affecting CYP2A6 activity and lung cancer risk. Cancer Res. 76, 5768–5776 (2016).
Gelernter, J. et al. Genome-wide association study of nicotine dependence in American populations: identification of novel risk loci in both African-Americans and European-Americans. Biol. Psychiatry 77, 493–503 (2015).
Zhang, J. et al. Genetic pleiotropy between nicotine dependence and respiratory outcomes. Sci. Rep. 7, 16907 (2017).
Wang, T. et al. Pleiotropy of genetic variants on obesity and smoking phenotypes: results from the Oncoarray Project of The International Lung Cancer Consortium. PLoS One 12, e0185660 (2017).
Akiyama, M. et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat. Genet. 49, 1458–1467 (2017).
Kanai, M. et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat. Genet. 50, 390–400 (2018).
Yang, J. et al. Genome-wide genetic homogeneity between sexes and populations for human height and body mass index. Hum. Mol. Genet. 24, 7445–7449 (2015).
Li, M. D., Cheng, R., Ma, J. Z. & Swan, G. E. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 98, 23–31 (2003).
Lindström, S. et al. Quantifying the genetic correlation between multiple cancer types. Cancer Epidemiol. Biomarkers Prev. 26, 1427–1435 (2017).
Nakajima, M. et al. A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nat. Genet. 46, 1012–1016 (2014).
How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General http://www.ncbi.nlm.nih.gov/pubmed/21452462 (CDC, 2010).
Weiss, N. S. Cigarette smoking and arteriosclerosis obliterans: an epidemiologic approach. Am. J. Epidemiol. 95, 17–25 (1972).
Powell, J. T. et al. Risk factors associated with the development of peripheral arterial disease in smokers: a case–control study. Atherosclerosis 129, 41–48 (1997).
Price, J. et al. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur. Heart J. 20, 344–353 (1999).
Rohrmann, S. et al. Smoking and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 108, 708–714 (2013).
Wang, S. et al. Significant associations of CHRNA2 and CHRNA6 with nicotine dependence in European American and African American populations. Hum. Genet. 133, 575–586 (2014).
Tsai, Y.-W., Tsai, T.-I., Yang, C.-L. & Kuo, K. N. Gender differences in smoking behaviors in an Asian population. J. Womens Health (Larchmt) 17, 971–978 (2008).
Speliotes, E. K. et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42, 937–948 (2010).
Hoffmann, T. J. et al. A large multiethnic genome-wide association study of adult body mass index identifies novel loci. Genetics 210, 499–515 (2018).
Berndt, S. I. et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat. Genet. 45, 501–512 (2013).
Shungin, D. et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 518, 187–196 (2015).
The International HapMap 3 Consortium Integrating common and rare genetic variation in diverse human populations. Nature 467, 52–58 (2010).
Li, Y., Willer, C. J., Ding, J., Scheet, P. & Abecasis, G. R. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet. Epidemiol. 34, 816–834 (2010).
Howie, B., Fuchsberger, C., Stephens, M., Marchini, J. & Abecasis, G. R. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat. Genet. 44, 955–959 (2012).
Abecasis, G. R. et al. An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012).
Loh, P.-R. et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat. Genet. 47, 284–290 (2015).
Aulchenko, Y. S., Struchalin, M. V. & van Duijn, C. M. ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinformatics 11, 134 (2010).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Lonsdale, J. et al. The Genotype-Tissue Expression (GTEx) Project. Nat. Genet. 45, 580–585 (2013).
Lamparter, D. et al. Fast and rigorous computation of gene and pathway scores from SNP-based summary statistics. PLoS Comput. Biol. 12, e1004714 (2016).
Ogata, H. et al. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 27, 29–34 (1999).
Kanehisa, M. et al. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 42, D199–D205 (2014).
Croft, D. et al. Reactome: a database of reactions, pathways and biological processes. Nucleic Acids Res. 39, D691–D697 (2011).
Nishimura, D. BioCarta. Biotech Softw. Internet Rep. 2, 117–120 (2001).
Acknowledgements
We acknowledge all patients who participated in the study. We thank the staff of the BBJ for their collecting and managing of clinical information and samples. We also thank the contributions of the Tohoku Medical Megabank Project, the Japan Public Health Center-based Prospective (JPHC) Study, the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study, and the Genetic Study Group of Investigation Committee on Ossification of the Spinal Ligaments for the case–control studies used in this study. This research was supported by the Tailor-Made Medical Treatment Program (the BioBank Japan Project) of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), and the Japan Agency for Medical Research and Development (AMED) (grant ID: JP17km0305002). N.I. and M.I. were funded by the AMED under JP18dm0107097, JP18km0405201 and JP18km0405028. S.I. was funded by the AMED under JP18ek0109223 and JP18bm0804006. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
N.M., M.A. and Y.K. contributed to the study concept and design. K.M., M.H. and M.Kubo collected and managed the BBJ samples. Y.M. and M.Kubo performed the genotyping. N.M., M.A., K.I., M.Kanai and A.T. performed the statistical analysis. S.I. M.I. and N.I. contributed to data acquisition. N.M. drafted the primary manuscript along with M.A., Y.O. and Y.K. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–7, Supplementary Tables 1–6 and 9–13, and Supplementary References.
Supplementary Tables
Supplementary Tables 7, 8 and 14–16
Rights and permissions
About this article
Cite this article
Matoba, N., Akiyama, M., Ishigaki, K. et al. GWAS of smoking behaviour in 165,436 Japanese people reveals seven new loci and shared genetic architecture. Nat Hum Behav 3, 471–477 (2019). https://doi.org/10.1038/s41562-019-0557-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-019-0557-y